Connected Health Market Trends and Insights. Most Popular Smart Medical Devices of 2021 by MedM
June 16, 2021
As a software company, MedM is uniquely positioned at the center of the Connected Health ecosystem, working closely with the manufacturers and distributers of portable medical devices, RPM service providers, system integrators, caregivers, and end-users. MedM believes that this ecosystem, inherently complex due to the multitude of its stakeholders and workflows, relies on interoperability for balance.
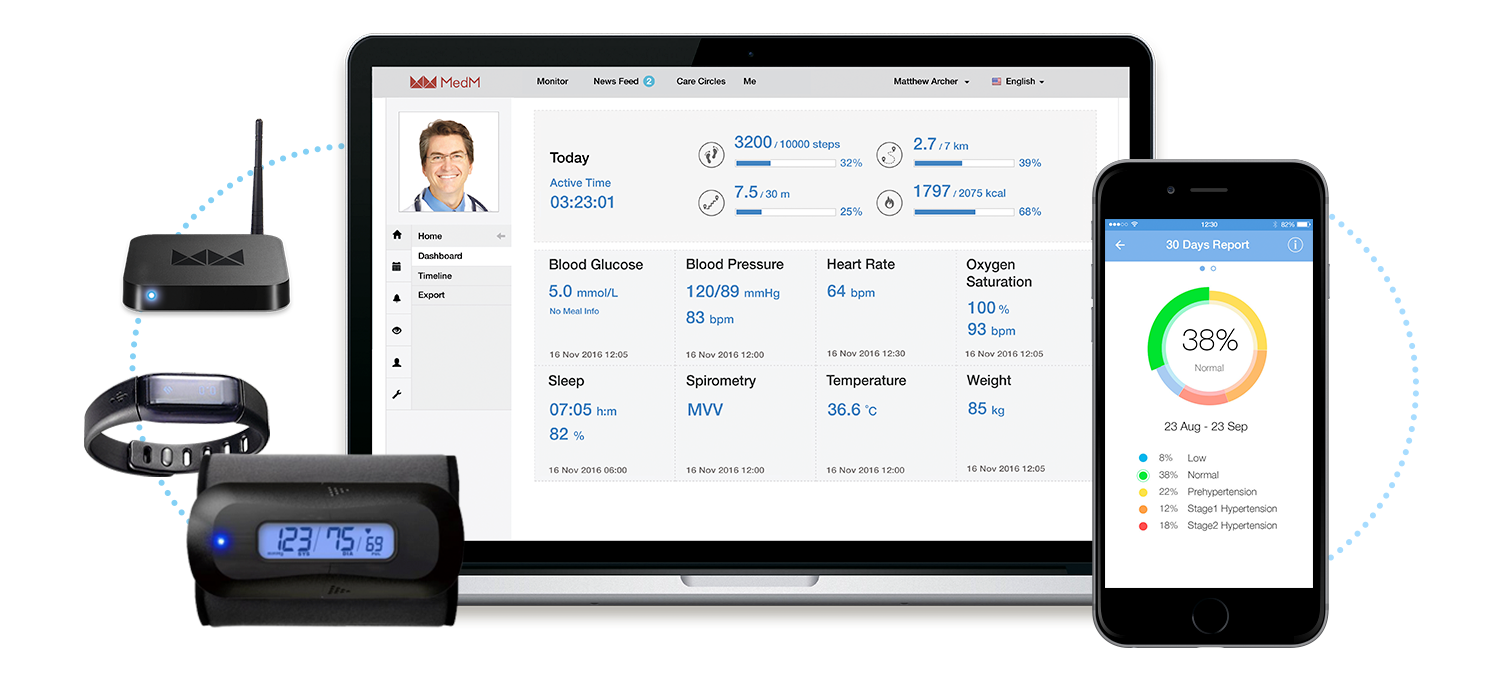
By virtue of its mission – Enabling Connected Health – MedM supports diversity and can integrate directly with a considerable percentage of all existing Bluetooth-enabled medical sensors. Not app-to-app or cloud-to-cloud sharing, but direct data collection from 450+ devices that patients already own and use. Thus, MedM’s secure and scalable technology can seamlessly deliver data from patients’ homes into existing caregiver workflows.
MedM’s corporate clients are based in the Americas, Europe, and Asia. Some of our customers work closely with national governments to prevent complications and improve outcomes, by delivering 90% patient adherence levels, increased clinical capacity, and reduced readmissions to hospitals.
Consumer-facing MedM Health Cloud alone (excluding the data from portals operated by our RPM customers), stores 13 million measurements. More than 8.5 million of them are automatically collected from hundreds of thousands of unique smart medical devices.
MedM Health Cloud Active Sessions:
| By Region | By Language |
| EU – 42% | English – 39% |
| USA – 23% | Russian – 24% |
| Russia – 19% | German – 7% |
| UK – 3% | Italian – 7% |
| Switzerland – 2% | Spanish – 4% |
| Ukraine – 2% | French – 4% |
| Canada – 2% | Polish – 3% |
| China – 1% | Chinese – 1% |
| Other – 6% | Other – 11% |
MedM’s software helps to log 16 different types of measurements (including vital signs) in 15 languages (with new languages added each year). End-users give our apps “two thumbs up” and the impossible “6 stars”, calling them “simple”, “functional”, “excellent”, and “the best way to consolidate data from smart devices by different manufacturers”. Consumers do appreciate the ease-of-use and ownership of data, but also “the best customer service” that MedM’s frontline support is able to offer. By attentively listening to what our customers want, MedM is able to quickly understand and introduce the most sought-after features, such as export from and import to Google Fit.
Smart Portable Medical Device Types by Popularity (Based on MedM Health Cloud Data)
1) Blood pressure monitors - 57%
2) Weight scales and weight scales with body composition - 30.8%
3) Blood glucose monitors - 5.5%
4) Thermometers – 3.6%
5) Heart rate monitors - 2%
6) Pulse oximeters - 0.8%
7) ECGs - 0.2%
8) Spirometers - 0.1%
Blood pressure monitors are also the most popular device category used by our RPM partners. Pulse oximeters are in the “fastest growing” category, with MedM’s OEM partners reporting a 6-fold increase in the capacity of production lines, and still not being able to meet the growing demand. As of summer 2021, the single most popular manufacturer of devices used by MedM B2C customers is Transtek.
The World’s Top 30 Leading Vendors of Smart Connected Medical Devices (in Alphabetical Order)
- A&D, Japan
- Andon Health, China
- Beurer, Germany
- Boso, Germany
- ChoiceMMed, China
- Contec, China
- D-Heart, Italy
- ForaCare, USA
- iHealth, USA
- Jumper, China
- Masimo, USA
- Medisana, Germany
- MIR, Medical International Research, Italy
- Nonin, USA
- Omron, Japan
- Oxitone, Israel
- Philips, Netherlands
- Radiant Innovation, Taiwan
- Raycome, China
- RENPHO, USA
- Roche, Switzerland
- Rossmax, Taiwan
- SD Biosensor, South Korea
- Sinocare, China
- TaiDoc, Taiwan
- Transtek, China
- Viatom, China
- Welch Allyn, USA
- Withings, France
- Xiaomi, China
Interoperability in 2021 and the Future of Connected Health
Up until 2020, the cautious conservatism of the healthcare industry has generally kept it from swiftly embracing the wonders of connectivity. Establishment of new standards, processes, regulations, and insurance practices is a costly and time-consuming effort. Precisely in response to the devastating challenges of the Covid-19 pandemic, many governments have dramatically increased their investment in healthcare innovation. While advances in connected wearable technology allow for a global shift in the healthcare paradigm towards prevention and care. RPM systems are being used to reinforce recovery of patients discharged from acute care units, to reduce the progression of chronic disorders, and to power global clinical trials.
With the demand for mobile monitoring devices continually growing manufacturers are reporting a worldwide semiconductor shortage. No single vendor is likely to reliably satisfy the needs of the budding RPM markets. Some strong brands - such as FitBit, iHealth, Withings, and Xiaomi - still favor a more isolated and stand-alone approach. However, industry-wide adoption of RPM with large-scale deployments can only be safely implemented if the use of monitoring devices is diversified amongst many suppliers. Moreover, the “bring your own device” or BYOD movement is further changing the face of healthcare, and the growing desire of patients to control their own health data is altering the way records are stored, accessed, and shared. Leading healthcare providers (85% of US hospitals) are already acknowledging this reality by adopting the FHIR protocol (Fast Healthcare Interoperability Resource protocol) for health data exchange.
Device diversity and interoperability both drive healthcare efficiency up and the costs down. MedM software solutions are designed to facilitate these aspects and we invite partners from many fields to join us on this journey together.